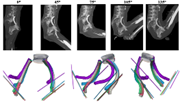
GST provides technical consulting, engineering testing services, and regulatory and grant support and conducts testing in ISO 13485-2016–certified laboratory and creative solutions in the field of medical device consulting. For 30 years, GST has provided intelligent solutions to our public and private sector customers, distinguishing GST as a leader and bringing our clients efficient, state-of-the-art science and technology services. An important part of our growing business is our Medical Division, which focuses on medical device consulting in the areas of device durability, biomechanical compatibility, medical image processing, clinical research, and regulatory strategy. We have served dozens of companies from startups to blue chips, assisting with medical device development from pre-design freeze to regulatory approval to post-market evaluation. Our medical research and technology experience, combined with our broader science expertise and stable company infrastructure, makes us an ideal teammate.